A Guide to Manufacturing Quality Management Systems
Explore how manufacturing quality management systems drive performance. Learn about core components, ISO standards, and technologies that...

Let’s be honest: in manufacturing, quality isn't just a goal; it's the entire game. A manufacturing quality management system (QMS) is the rulebook that turns quality from a hopeful outcome into a deliberate, repeatable process.
Think of it less as a rigid set of instructions and more as your factory’s central nervous system. It’s a living framework of policies, processes, and controls designed to make sure every single product meets—and exceeds—customer expectations, day in and day out. It’s about building quality in from the very first step, not just inspecting for defects at the end.
Understanding the Purpose of a Quality Management System
Imagine a production line where every part, every kit, and every final assembly is perfect. While 100% perfection is the dream, getting incredibly close with predictable consistency is the reality a great QMS delivers. It coordinates your people, processes, and technology toward one unified goal: reliability.
This isn't some dusty binder sitting on a shelf. A modern QMS is active. It dictates how raw materials are verified, how components are kitted and assembled, and how final products are tested before they ever leave the dock. By standardizing these critical functions, a QMS replaces guesswork with predictable, repeatable results.
From Reactive Fixes to Proactive Prevention
The old way of thinking about quality was reactive. You'd have inspectors at the end of the line, trying to catch mistakes before they got out the door. A modern QMS completely flips that script. It’s all about preventing defects from ever happening in the first place.
A well-implemented QMS shifts the focus from "finding and fixing" defects to "predicting and preventing" them. It’s about building quality into the process, not just inspecting it at the end.
This shift is a game-changer for your bottom line. When you embed quality checks throughout the production cycle—from supplier verification to in-process testing and sequencing—you spot issues early. And catching problems early means they are far cheaper and easier to fix.
To help you see how this works in practice, here are the core functions a QMS manages.
Core Functions of a Manufacturing QMS at a Glance
This table breaks down the primary functions of a modern QMS, helping you quickly grasp its scope and purpose in a real-world manufacturing environment.
Ultimately, a strong QMS is a strategic tool that does more than just catch errors. It’s designed to:
- Standardize Operations: Ensures every team member follows the same proven procedures for every task, whether it's kitting, sequencing, or warehousing.
- Drive Continuous Improvement: Creates a feedback loop for finding the root cause of problems and implementing corrective actions so they never happen again.
- Enhance Traceability: Builds a complete history for every part, making it easy to trace any component back to its source if an issue ever comes up.
- Ensure Compliance: Provides the documented proof needed to meet tough industry standards and satisfy audits from OEM or Tier 1 customers.
For a partner like Wolverine Assemblies, our QMS is our documented promise. It's the system that guarantees every kit, sub-assembly, and shipment we handle meets the highest standards—no exceptions.
The Seven Pillars of an Effective QMS
A strong manufacturing QMS isn’t just one thing—it’s a structure built on seven interconnected pillars. When they all work together, they create a framework that stops you from just catching defects and starts preventing them altogether.
Think of these pillars less like separate departments and more like integrated functions that build the foundation for operational excellence. Each one supports the others, making sure quality is the top priority from the moment raw materials arrive to the second the final product ships.
This is how abstract goals turn into concrete actions on the factory floor. The diagram below shows how the core inputs—people, process, and technology—are brought together by a QMS to hit the ultimate goal: consistent quality.
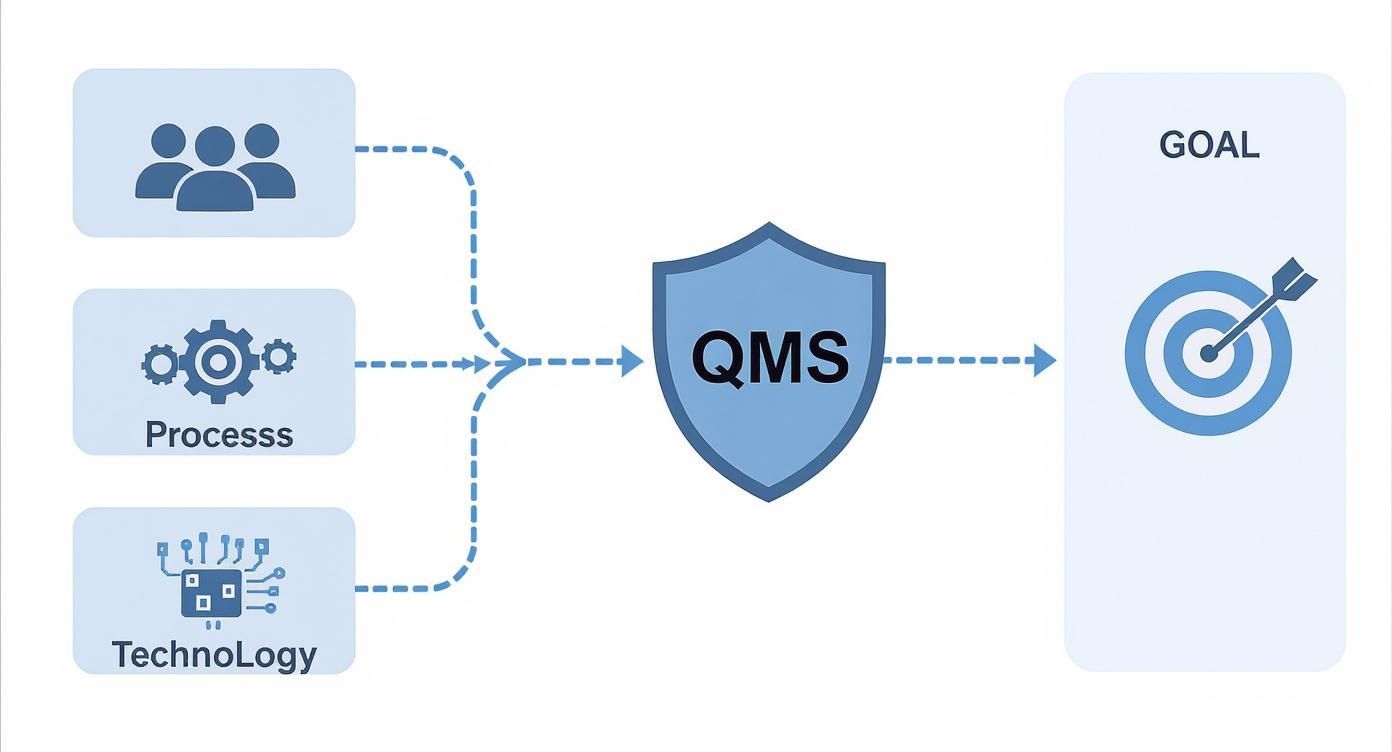
A QMS acts as the central system that organizes every resource toward that one objective. Let's break down the seven pillars that make it all happen.
Process Control and Inspection
The first two pillars, Statistical Process Control (SPC) and Inspection & Testing, are your front line of defense against defects. SPC is all about using data to monitor a process, helping you predict and stop issues before they even start. It’s like a smoke detector for your production line—it warns you of a problem long before a fire breaks out.
Inspection and testing are the hands-on verification steps. This isn’t just a quick check at the end of the line. It involves:
- Receiving Inspection: Making sure incoming parts from suppliers meet your exact specs.
- In-Process Checks: Testing during assembly or kitting to confirm each step is done right.
- Final Inspection: Validating that the finished product meets all customer requirements before it leaves the building.
Together, they make sure quality is built-in, not just inspected-on.
Managing Deviations and Finding Solutions
No matter how tight your processes are, things can go wrong. That’s where Nonconformance Management and Corrective and Preventive Actions (CAPA) come in.
The second a part or product fails inspection, the nonconformance process kicks in. The goal is simple: identify, document, and contain the problem so it never reaches the customer. This could mean quarantining a bad batch of components or holding a shipment of kitted assemblies.
But just containing a problem isn’t good enough. The CAPA process takes it a step further and acts as the detective for your QMS.
The real job of CAPA is to get past the symptom and find the root cause of a quality issue. A corrective action fixes today's problem. A preventive action makes sure it never happens again.
For example, if a component keeps getting put in the wrong bin during sequencing, a corrective action is to retrain the operator. A preventive action is to redesign the workstation so it’s physically impossible to make that mistake.
Ensuring Quality Across the Supply Chain
Your quality is only as strong as your weakest link—and that's often in your supply chain. This is why Supplier Quality Management is non-negotiable. It’s a systematic approach to picking, evaluating, and monitoring your suppliers to guarantee their parts are up to your standards.
This pillar is critical for services like ours at Wolverine Assemblies, where incoming components must be perfect for our kitting and sub-assembly work. It’s about partnering with suppliers who are just as committed to quality as you are.
Just as important is Documentation and Record Control. This pillar is the official biography of every product, tracking every step of its journey. Accurate records are essential for audits, troubleshooting, and proving you comply with standards like ISO 9001.
Finally, the seventh pillar is Internal and External Audits. Audits are systematic check-ups to verify your QMS is actually working. Internal audits are self-checks to find areas for improvement, while external audits are done by customers or certification bodies to confirm compliance.
These audits aren't about finding fault; they're about finding opportunities. They are a core part of the feedback loop that drives growth. To see how this fits into a bigger picture, you can learn more about embedding continuous improvement in manufacturing in our detailed guide.
Navigating Key Quality Standards and Compliance
Think of quality standards as the shared language of manufacturing. They aren't just arbitrary rules; they are proven blueprints that guide a manufacturing quality management system toward peak performance. When everyone in the supply chain speaks this same language, you get reliability and precision.
Adhering to these frameworks is a powerful signal to customers and regulators. It shows you’re committed to processes that are documented, repeatable, and completely focused on the end customer. Getting a certification isn't just for a plaque on the wall—it's a commitment to a culture of excellence.
The Global Benchmark: ISO 9001
At the core of global quality is ISO 9001. It’s the foundational standard for just about any industry, setting the essential criteria for a QMS. You can think of it as the operating system for quality, built on a few key principles that create a resilient business.
- Customer Focus: Everything you do is geared toward meeting and exceeding what your customer needs.
- Leadership Commitment: Top management isn’t just aware of the QMS; they are actively driving it.
- Process Approach: All activities are managed as interconnected processes to deliver consistent, predictable results.
- Evidence-Based Decision Making: You use real data and analysis to make smart choices, not just guesses.
- Continuous Improvement: There’s a built-in system for constantly finding and acting on ways to get better.
Implementing ISO 9001 gives a company a solid, reliable framework for managing every part of its operation, from the initial order to the final delivery.
The Automotive Standard: IATF 16949
For any company in the automotive supply chain, IATF 16949 is the gold standard. It takes the foundation of ISO 9001 and adds a layer of strict requirements specific to automotive design, development, production, and service. For OEM and Tier 1 suppliers, it’s not optional—it’s mandatory.
IATF 16949 is laser-focused on one thing: preventing defects. It’s all about reducing variation and waste everywhere in the supply chain. This means rigorous risk management, solid contingency plans, and meticulous control over every single process.
This intense focus is vital for partners like Wolverine Assemblies, where our kitting and sequencing services feed directly into an OEM's production line. For any business aiming for international recognition, understanding global compliance certification is a must for navigating these complex standards.
The High Cost of Non-Compliance
Ignoring or half-heartedly implementing these standards is a huge gamble. Non-compliance isn't just a procedural slip-up; it comes with severe financial and reputational damage that can cripple a business. The fallout can include staggering recall costs, regulatory fines, lost contracts, and a permanent loss of customer trust.
The stakes are incredibly high. Some manufacturing sectors are facing a crisis, with a 115% increase in recalls since 2018 and total costs hitting $5 billion annually. Adhering to standards isn't a box-ticking exercise—it’s a powerful strategic advantage that protects your brand and your bottom line. An effective quality system is also a core part of operational excellence, and you can learn more by checking out our guide on how to improve supply chain efficiency.
Integrating Your QMS with Modern Technology
In today's fast-paced manufacturing world, a quality management system stuck on paper is like trying to compete in a Formula 1 race with a go-kart. It just can't keep up. To stay competitive, modern manufacturing quality management systems need to be wired directly into the technology that runs the facility. This connection turns a static rulebook into a dynamic, real-time command center for quality.
The digital backbone for this integration is almost always an Enterprise Resource Planning (ERP) platform. Think of it as the central nervous system for the entire operation, linking quality data with everything else—production schedules, inventory levels, and the entire supply chain. You can get a deeper dive into these platforms in our article on what an ERP system is in manufacturing.

The Power of a Centralized Platform
Integrated platforms like the PLEX Smart Manufacturing Platform are game-changers because they create a single source of truth. When a nonconformance is flagged on the shop floor, that alert is instantly visible to the purchasing, planning, and management teams. There are no information delays, no data silos, and no excuses.
This real-time visibility leads to faster, smarter decisions. For example, if a batch of incoming material fails inspection, the system can automatically place a hold on all related stock. This simple action prevents faulty components from ever making it into our kitting or assembly workflows, stopping a major quality issue before it even starts.
Seamless Communication and Traceability
Technology also completely overhauls how manufacturers talk to their suppliers. Tools like Electronic Data Interchange (EDI) and Advanced Shipping Notices (ASN) automate the conversation, ensuring everyone is on the same page.
An ASN, for instance, is a digital heads-up that tells our receiving facility exactly what’s in a shipment before the truck even backs into the dock. This powerful little tool delivers huge benefits:
- Improved Receiving Efficiency: Our warehouse team knows what to expect, preparing for faster unloading and verification.
- Enhanced Traceability: Part and lot numbers are captured digitally the moment they arrive, creating an instant and accurate record.
- Proactive Quality Checks: We can review incoming component data against our quality specs ahead of time.
This seamless data flow means quality control starts long before parts ever hit the production floor, building a stronger, more transparent supply chain from the ground up.
The Shift to Predictive Quality with AI
The biggest technological leap for QMS, however, is the arrival of artificial intelligence (AI) and machine learning. These systems are flipping the old quality model on its head, moving from a reactive "find-and-fix" approach to a proactive "predict-and-prevent" powerhouse.
Instead of just catching defects after they happen, AI analyzes massive streams of production data—from machine sensor readings to workshop temperatures—to spot the subtle patterns that come before a quality failure.
By using AI, manufacturers can see trouble coming and make process adjustments in real-time. This predictive power helps prevent scrap, rework, and the costly downtime that kills profitability.
The impact is massive. Real-time anomaly detection systems powered by AI are on track to slash defects by 30% in 2025. Even better, AI-driven predictive maintenance can cut equipment downtime by 50% while trimming maintenance costs by 40%. By building smart technology into its core, a modern QMS becomes an organization's most powerful tool for hitting its operational goals.
How to Measure QMS Success with the Right KPIs
A manufacturing QMS is only as good as the results it delivers. But without the right data, you're just flying blind, completely unable to tell if your efforts are driving real improvement or just adding complexity. Key Performance Indicators (KPIs) are the instruments on your dashboard, turning abstract quality goals into tangible, measurable metrics that guide your decisions.
The old saying, "If you can't measure it, you can't improve it," is the absolute truth here. KPIs give you objective proof of your QMS's health. They show you where you’re strong, pinpoint exactly where you’re weak, and highlight the best opportunities for continuous improvement. It’s how you move from guesswork to a data-driven strategy for operational excellence.

Essential KPIs for the Factory Floor
While every facility has its own unique challenges, a few core KPIs are universally critical for measuring any manufacturing QMS. Think of these as the vital signs for your operation’s efficiency, waste, and customer satisfaction.
First Pass Yield (FPY): This is the gold standard. FPY tracks the percentage of products made correctly the very first time, with no need for rework or repairs. A high FPY score is a clear sign of a stable, capable, and efficient production process.
Scrap Rate: This metric tells you exactly what percentage of materials are being thrown away during production. A high scrap rate is a direct hit to your bottom line, pointing to problems with machinery, processes, or even the quality of your raw materials.
Customer Rejection Rate (PPM): Often measured in Parts Per Million (PPM), this KPI tracks how many units customers send back. It's the ultimate verdict on how your quality is perceived in the real world and has a direct impact on your brand's reputation.
Cost of Quality (COQ): This is a powerful financial metric that balances the costs of preventing defects (good quality) against the costs of fixing them (poor quality). COQ helps justify investments in your QMS by showing how proactive measures end up saving you from much more expensive failures down the road.
Leading vs. Lagging Quality Indicators
Not all metrics tell the same story. To build a truly forward-looking quality dashboard, you have to understand the difference between two types of indicators: leading and lagging. Lagging indicators report on what already happened, while leading indicators help you predict what's coming next.
Think of it this way: Lagging indicators, like Customer Rejection Rate, tell you the final score of a game that's already over. Leading indicators, like operator training completion rates, help you predict the outcome of the next game. A balanced dashboard needs both.
Understanding the difference between predictive (leading) and historical (lagging) metrics is key to a proactive QMS. This table breaks it down.
| Leading vs. Lagging Quality Indicators || :--- | :--- | :--- || Indicator Type | Definition | Example KPIs || Leading Indicators | Predictive metrics that measure activities designed to prevent future quality problems. | Employee training hours, number of preventive maintenance tasks completed, supplier audit scores. || Lagging Indicators | Historical metrics that report on outcomes and past performance. | First Pass Yield, scrap rate, number of warranty claims, customer complaints. |
At the end of the day, quality is about the end user. That's why connecting internal metrics to external satisfaction is so critical. A great QMS is one that not only improves factory floor numbers but also understands how to go about measuring customer experience effectively.
By tracking a balanced mix of these KPIs, you can turn your QMS from a simple compliance checklist into a powerful engine for driving real business results.
A Practical Roadmap for QMS Implementation
Putting a manufacturing quality management system in place can feel like a massive undertaking. The goal—a culture of consistent quality—is clear, but the path to get there can seem complicated and full of potential wrong turns. But a successful rollout isn't one giant leap; it's a series of deliberate, well-planned steps.
The journey starts not on the factory floor, but in the boardroom. The single biggest predictor of success is unwavering leadership commitment. Without buy-in from the top, any QMS initiative is seen as just another temporary project that will eventually lose steam and resources.
Once leadership is on board, the next step is to get an honest look at where you are right now. This means a thorough gap analysis, comparing your current processes against the requirements of your chosen standard, like ISO 9001. This isn't about finding fault; it's about spotting opportunities and creating a realistic project scope.
Building Your Implementation Framework
With a clear starting point, you can structure the process around the proven Plan-Do-Check-Act (PDCA) cycle. This framework breaks the huge task into a manageable, continuous loop. It ensures the QMS becomes a living part of your operation, not just a binder of procedures sitting on a shelf.
- Plan: Define your quality policy, set clear objectives, and assign roles. This is where you document new procedures and build the core of your QMS manual.
- Do: Roll out the plan. This phase is all about communication and training. Every employee needs to understand not just what they need to do differently, but why it matters to them and the customer.
- Check: This is where you measure your progress. You'll conduct your first internal audits and start tracking the KPIs you established. Are the new processes actually being followed? Are they delivering the results you expected?
- Act: Based on what you find, you make adjustments. Use data from audits and KPIs to refine processes, address nonconformances, and drive corrective actions. This is the engine of continuous improvement.
This cycle isn't a one-and-done event. It's the rhythm that keeps the QMS alive and effective long after the initial implementation is over.
Avoiding Common Implementation Pitfalls
Many QMS implementations stumble, but not because of the system itself. It's usually due to entirely avoidable mistakes. Knowing these common pitfalls from the start can be the difference between a successful launch and a frustrating failure.
The goal of implementation is not just to get a certificate on the wall. It's to build a sustainable culture where every team member takes ownership of quality. Treating it as a one-time project is the fastest way to ensure it fails.
Here are the most common traps to watch out for:
- Poor Communication: Failing to explain the "why" behind the QMS. If employees see it as just more paperwork, they won't engage.
- Inadequate Training: Simply handing someone a new procedure and calling it "training" doesn't work. Real training ensures deep understanding and competence.
- Lack of Resources: Underestimating the time, budget, and people needed to implement and maintain the system properly.
- Ignoring Employee Feedback: The people doing the work often have the best insights. A QMS that doesn't listen is a QMS that won't last.
By securing strong leadership, following a structured roadmap, and actively avoiding these common traps, you can turn a daunting task into a clear path toward building a lasting culture of quality.
Common Questions About Manufacturing QMS
Once you get the basics down, the real-world questions about a manufacturing quality management system start to pop up. Think of this as the practical FAQ—the stuff you wonder about when you're actually trying to make it work on the shop floor.
Here are a few of the most common questions we hear, with straightforward answers.
How Long Does It Take to Implement a QMS?
There’s no magic number here. The timeline depends on your company's size, how complex your operations are, and what processes you already have in place. For a small to mid-sized manufacturer starting from nearly scratch, getting certified for ISO 9001 typically takes 6 to 12 months.
But this can move faster if your procedures are already well-documented and leadership is fully on board. The goal isn’t speed, though—it’s building a system that actually works and lasts. Rushing it is a classic mistake that leaves you with a QMS that only exists on paper.
Is a QMS Only for Large Corporations?
Absolutely not. It’s easy to think of QMS as something only for massive OEMs and Tier 1 suppliers, but the core ideas help businesses of any size. A scaled-down, practical QMS helps smaller companies work smarter, cut waste, and build a name for being reliable.
A QMS is scalable. A small shop doesn't need the bureaucracy of a global giant, but it still benefits immensely from standardized work, fixing problems systematically, and keeping customers happy. The principles are the same, just applied differently.
What Is the Biggest Challenge in Maintaining a QMS?
Getting the system set up is one thing. The real challenge is keeping it going and preventing it from becoming a dusty binder on a shelf. After the big push for certification, it's easy to slip into "compliance mode," where you’re just doing the bare minimum to pass the next audit.
The only way to avoid this is to build a true culture of quality. This isn’t just a buzzword; it means:
- Keeping everyone involved: Quality isn't one person's job. It's about empowering your whole team to spot issues and suggest improvements.
- Leadership that lives it: Management has to visibly support and use the QMS, not just talk about it.
- Using data to get better: KPIs and audit findings should be tools for real change, not just paperwork to be filed away.
A healthy QMS is a living part of your business. It evolves as you grow, constantly pushing you to be more efficient and deliver better results.
At Wolverine Assemblies, LLC, our QMS is the engine behind every kitting, assembly, and warehousing project we manage. See how our commitment to quality can make your supply chain stronger by visiting us at https://www.wolverine-llc.com.
Subscribe to our weekly newsletter

.png)



